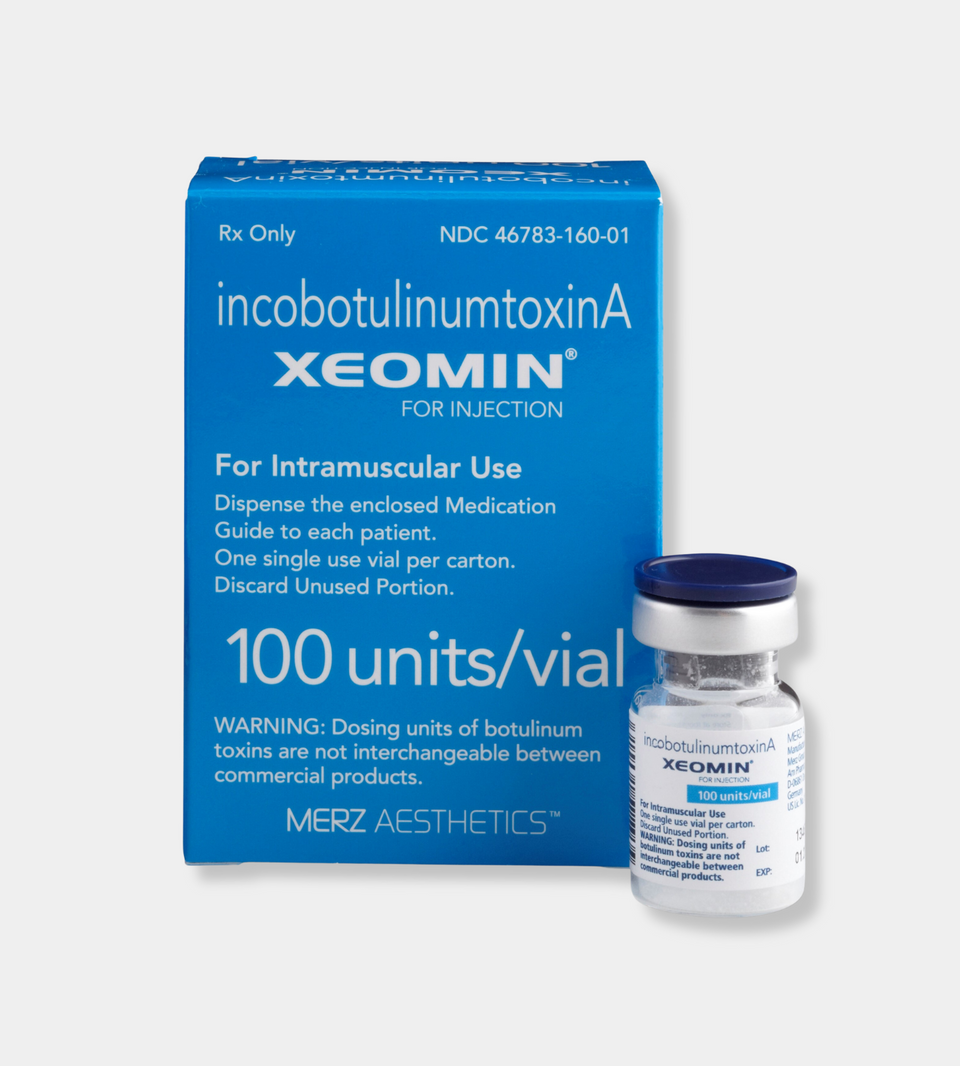
Description
Xeomin (IncobotulinumtoxinA) is an FDA-approved neuromodulator used for cosmetic and medical purposes, primarily to reduce wrinkles, fine lines, and dynamic facial folds. Unlike other botulinum toxin products, Xeomin is a highly purified formulation that contains no complexing proteins, which may reduce the risk of antibody formation and ensure consistent results.
Key Benefits of IncobotulinumtoxinA:
Smooths forehead lines, frown lines, and crow’s feet
FDA-approved and clinically tested for safety
Long-lasting results (typically 3–4 months)
Minimal downtime with professional administration
Reduced risk of immune response compared to other botulinum products
| Property | Details |
|---|---|
| Product Name | Xeomin |
| Form | Injectable solution (vial) |
| Type | Botulinum toxin type A (cosmetic neurotoxin) |
| Strength | 100 units per vial |
| Usage | Temporarily reduces the appearance of facial wrinkles and fine lines |
| Suitable For | Adults seeking cosmetic wrinkle treatment under licensed professional supervision |
| Shelf Life | Typically 2–3 years (check packaging) |
| Storage | Store refrigerated (2–8°C); do not freeze; protect from light |
| Packaging | Sterile vial with reconstitution instructions |









Reviews
There are no reviews yet.